Example: Absolute Solvation Free Energy
Jump to navigation
Jump to search
| Free Energy Fundamentals |
|---|
 |
|
Methods of Free Energy Simulations
|
| Free Energy How-to's |
|---|
 |
This page will guide you through the process of designing a simple free energy simulation. In particular, this example will set up an OPLS-AA forcefield simulation of ethanol in a box of TIP3P water. The purpose of this page is not to take you through step-by-step the process of simulation, but instead guide you through the logical decisions that can be applied to any simulation package of your choosing.
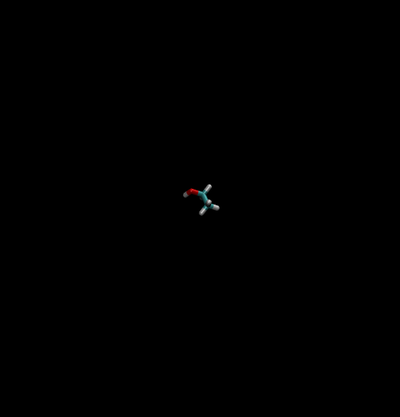
Lets start with the visuals of the system, we are want to simulate a box of TIP3P water where an ethanol molecule is appeared inside the box.

|
What is the Thermodynamic Cycle?
The first thing we must do is define which path we will carry out our simulation under. There are two main paths we could take:
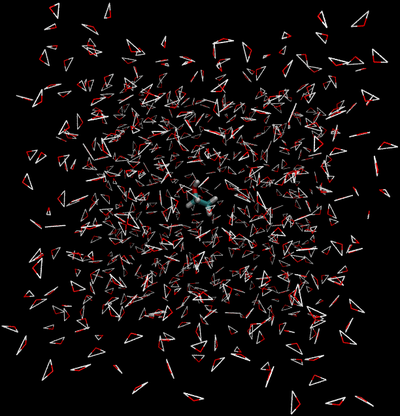
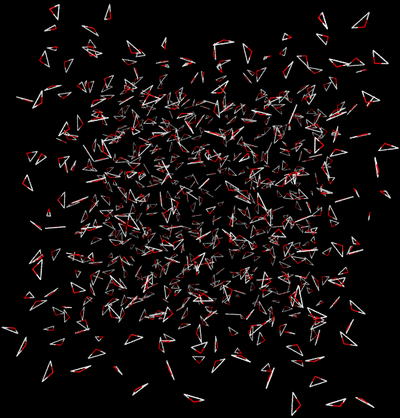
- Direct Caclulation: Intermolecular interactions are eliminated between the water and ethanol.
- End State A) Fully interacting system where the ethanol is interacting with every water.
- End State B) Ethanol interacts with itself and is free to move around the box as if the water was not there, and water interacts with itself and acts as if the ethanol is not there.
- Effectively, the interactions would look like the following between the two states
|

|
|

|
|
| State A | State B | |||
- Indirect Calculation: Disable ALL the ethanol interactions, then do it again in vacuum so you can add the forces back into the calculation. Recall that intramolecular interactions must remain on to be accurate.