Difference between revisions of "Example: Absolute Solvation Free Energy"
(Created page with "{{fundamentals | cTopic=Examples}} This page will guide you through the process of designing a simple free energy simulation. In particular, this example will set up an OPLS-AA ...") |
m |
||
| Line 14: | Line 14: | ||
The first thing we must do is define which path we will carry out our simulation under. There are two main paths we could take: | The first thing we must do is define which path we will carry out our simulation under. There are two main paths we could take: | ||
| − | + | == Direct Caclulation== | |
| − | + | Intermolecular interactions are eliminated between the water and ethanol. | |
| − | + | * ''End State A)'' Fully interacting system where the ethanol is interacting with every water. | |
| − | + | * ''End State B)'' Ethanol interacts with '''itself''' and is free to move around the box as if the water was not there, and water interacts with '''itself''' and acts as if the ethanol is not there. | |
| − | + | *: Effectively, the interactions would look like the following between the two states | |
| + | |||
{| | {| | ||
| [[File:TIP3P-Ethanol.png|400px]] | | [[File:TIP3P-Ethanol.png|400px]] | ||
| Line 29: | Line 30: | ||
| | | | ||
| colspan="3" align="center" | <span style="font-size:200%">State B</span> | | colspan="3" align="center" | <span style="font-size:200%">State B</span> | ||
| − | |} | + | |} |
| − | + | ||
| + | ==Indirect Calculation== | ||
| + | Disable ALL the ethanol interactions, then do it again in vacuum so you can add the forces back into the calculation. Recall that [[Thermodynamic State#Constructing a Thermodynamic Path| ''intramolecular'' interactions must remain on]] to be accurate. For this, we need four total states, | ||
| + | * ''End State I-A)'' Fully interacting system where the ethanol is interacting with the water. | ||
| + | * ''End State I-B)'' Ethanol is annihilated leaving '''only''' water to interact with itself. | ||
| + | * ''End State II-A)'' Vacuum system where ethanol is allowed to interact with itself. | ||
| + | * ''End State II-B)'' Ethanol is annihilated in vacuum, leaving no interactions. | ||
| + | *: Visually: | ||
| + | |||
| + | {| | ||
| + | | [[File:TIP3P-Ethanol.png|400px]] | ||
| + | | [[File:Doublearrow.png|100px]] | ||
| + | | [[File:Waterbox.png|400px]] | ||
| + | |- | ||
| + | | align="center" | <span style="font-size:200%">State I-A</span> | ||
| + | | | ||
| + | | align="center" | <span style="font-size:200%">State I-B</span> | ||
| + | |- | ||
| + | | colspan="3" bgcolor="gray" | | ||
| + | |- | ||
| + | | [[File:Ethanol_in_vac.png|400px]] | ||
| + | | [[File:Doublearrow.png|100px]] | ||
| + | | [[File:Blackbox.png|400px]] | ||
| + | |- | ||
| + | | align="center" | <span style="font-size:200%">State II-A</span> | ||
| + | | | ||
| + | | align="center" | <span style="font-size:200%">State II-B</span> | ||
| + | |} | ||
| + | |||
| + | =What are the End States?= | ||
| + | The end states are shown above and depend on the thermodynamic cycle chosen. One may ask "why would you ever choose the Indirect calculation?" since it requires seemingly more steps. The answer will usually depend on the software you are using. Free energy calculations are not always implemented in code and the software may not be able to define a system with only selective interactions (i.e. you get either all atoms interacting together or you get none). | ||
| + | |||
| + | Even if you must do the annihilation runs, vacuum simulations with one molecule are often very quick and efficient, so there is a negligible increase in total computation time. | ||
| + | |||
| + | =What are the [[Intermediate States]]?= | ||
| + | Defining the intermediate states are one of the most challenging parts of any free energy calculation, and each method shown above will have different (although very similar) states. | ||
| + | |||
| + | For the '''Direct Calculation,''' a [[Intermediate_States#Intermolecular_Forces|robust way]] would be to: | ||
| + | * Linearly turn off intermolecular charge interactions of the ethanol with the solvent. | ||
| + | * Scale the van der Waals interactions from ethanol to the solvent by a [[Intermediate_States#Soft_Core_Potentials|soft core potential]]. | ||
| + | |||
| + | For the '''Indirect Calculation,''' a slightly different but equally robust scheme would be: | ||
| + | * Linearly scale the charges on ethanol to zero (Note: this fully disables the charge as opposed to remove interacting with only one type of molecule). | ||
| + | * Turn off the Lennard-Jones interactions with a soft core transformation. | ||
| + | * Repeat this process in vacuum. | ||
Revision as of 10:34, 10 October 2012
| Free Energy Fundamentals |
|---|
 |
|
Methods of Free Energy Simulations
|
| Free Energy How-to's |
|---|
 |
This page will guide you through the process of designing a simple free energy simulation. In particular, this example will set up an OPLS-AA forcefield simulation of ethanol in a box of TIP3P water. The purpose of this page is not to take you through step-by-step the process of simulation, but instead guide you through the logical decisions that can be applied to any simulation package of your choosing.
Lets start with the visuals of the system, we are want to simulate a box of TIP3P water where an ethanol molecule is appeared inside the box.

|
What is the Thermodynamic Cycle?
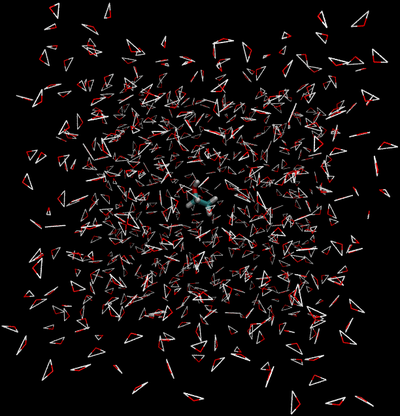
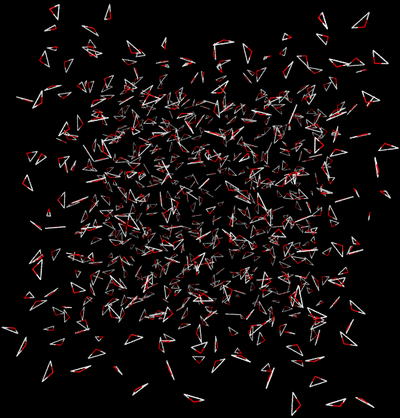
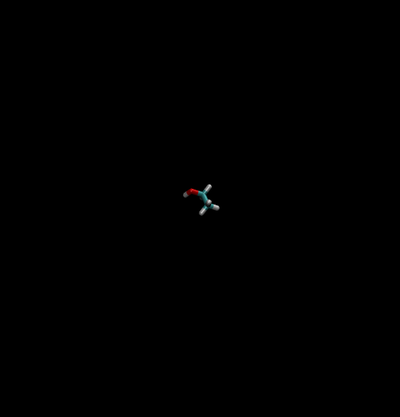
The first thing we must do is define which path we will carry out our simulation under. There are two main paths we could take:
Direct Caclulation
Intermolecular interactions are eliminated between the water and ethanol.
- End State A) Fully interacting system where the ethanol is interacting with every water.
- End State B) Ethanol interacts with itself and is free to move around the box as if the water was not there, and water interacts with itself and acts as if the ethanol is not there.
- Effectively, the interactions would look like the following between the two states
|

|
|

|
|
| State A | State B | |||
Indirect Calculation
Disable ALL the ethanol interactions, then do it again in vacuum so you can add the forces back into the calculation. Recall that intramolecular interactions must remain on to be accurate. For this, we need four total states,
- End State I-A) Fully interacting system where the ethanol is interacting with the water.
- End State I-B) Ethanol is annihilated leaving only water to interact with itself.
- End State II-A) Vacuum system where ethanol is allowed to interact with itself.
- End State II-B) Ethanol is annihilated in vacuum, leaving no interactions.
- Visually:

|

|

|
| State I-A | State I-B | |

|

|

|
| State II-A | State II-B | |
What are the End States?
The end states are shown above and depend on the thermodynamic cycle chosen. One may ask "why would you ever choose the Indirect calculation?" since it requires seemingly more steps. The answer will usually depend on the software you are using. Free energy calculations are not always implemented in code and the software may not be able to define a system with only selective interactions (i.e. you get either all atoms interacting together or you get none).
Even if you must do the annihilation runs, vacuum simulations with one molecule are often very quick and efficient, so there is a negligible increase in total computation time.
What are the Intermediate States?
Defining the intermediate states are one of the most challenging parts of any free energy calculation, and each method shown above will have different (although very similar) states.
For the Direct Calculation, a robust way would be to:
- Linearly turn off intermolecular charge interactions of the ethanol with the solvent.
- Scale the van der Waals interactions from ethanol to the solvent by a soft core potential.
For the Indirect Calculation, a slightly different but equally robust scheme would be:
- Linearly scale the charges on ethanol to zero (Note: this fully disables the charge as opposed to remove interacting with only one type of molecule).
- Turn off the Lennard-Jones interactions with a soft core transformation.
- Repeat this process in vacuum.